Digital transformation in the Life Sciences industry involves weaving digital technologies throughout every facet of a business, reshaping its operations, and enhancing the value offered to customers.
It is more than a technological shift; it’s a cultural shift. It demands that businesses question established norms and try new things, fundamentally changing how organizations operate and deliver value to customers.
One solution leading the charge is Exeevo’s next-generation CRM, Omnipresence. Built on the architecture of Microsoft Dynamics 365, this solution takes Life Sciences digital transformation to an unprecedented level. Recognized for the past five years by Gartner, IDC, and Everest as an alternative to Salesforce-based systems. Omnipresence is a next-gen, unified, intelligent CRM platform that brings Sales, Medical Affairs, Marketing Automation, Events Management, Content Management, and powerful BI/ML/AI capabilities from the same solution. It caters to today’s needs while anticipating tomorrow’s challenges.
Let’s explore digital transformation in Life Sciences in more detail and how technology can help companies overcome the challenges related to it.
How Digital Transformation Changes the Life Sciences Industry?
Digital transformation in Life Sciences can change healthcare and biomedical research. Enhanced computational capabilities and data-driven analytics can lead to many new developments. Let’s look at the numerous benefits of digital transformation in Life Sciences.
Digital Ecosystem
Digital transformation helps create a digital ecosystem within the Life Sciences industry. This ecosystem comprises interconnected
technologies and data-sharing platforms that enhance collaboration and fuel innovation. For example,
- Cloud-based platforms enable real-time collaboration
- Artificial intelligence algorithms swiftly analyze vast amounts of data
- High-throughput sequencing technologies expedite genetic research
Components such as cloud computing, IoT devices, and other emerging technologies are interconnected in the digital ecosystem. They promote seamless data exchange and collaborative efforts that lead to accelerated discoveries and better patient care outcomes.
Effectiveness
Advanced analytics, AI, and machine learning improve decision-making, with real-time data insights and predictive analytics enabling proactive actions.
For instance, pharmaceutical companies like Roche leverage artificial intelligence to enhance the effectiveness of clinical trials and accelerate the drug discovery process. It reduces the time and cost of bringing new treatments to market.
Efficiency
Digital transformation significantly enhances the efficiency of operations within the Life Sciences industry in the following ways:
- Automation: It reduces manual processes, minimizes errors, and increases productivity. For instance, automated laboratory processes expedite research and reduce human error.
- Streamlined workflows: They enable quicker data analysis and decision-making. Integrated digital platforms can unite diverse datasets and enhance collaborative research.
- Optimized resource allocation: Data-driven decision-making helps in optimizing resource allocation. It helps in better prioritization and management of projects and funds.
For example, Pfizer used AI to run trials, make vaccines, and improve distribution. They invested in digital systems and modernized research operations. This sped up vaccine production and distribution during the early stages of the global pandemic.
Key Takeaway:

CIOs in the life sciences industry must assess their existing technology infrastructure, identify gaps and prioritize initiatives aligned with organizational goals to develop an effective strategic framework for digital transformation. By focusing on critical areas such as data management and analytics, cloud computing, AI, machine learning, IoT, and cybersecurity organizations can create a comprehensive roadmap for their digital journey.
Digital Twin
Digital twins are virtual replicas of physical systems or processes. They enable researchers to simulate experiments, predict future outcomes, and optimize procedures without the risk and cost of real-world trials.
Digital twin technology allows experts in the Life Sciences to create and examine virtual models of patient conditions in great detail. These capabilities lead to quicker findings and improved results that can save costs. Here’s how digital twin implementation contributes to the ROI in the long run.
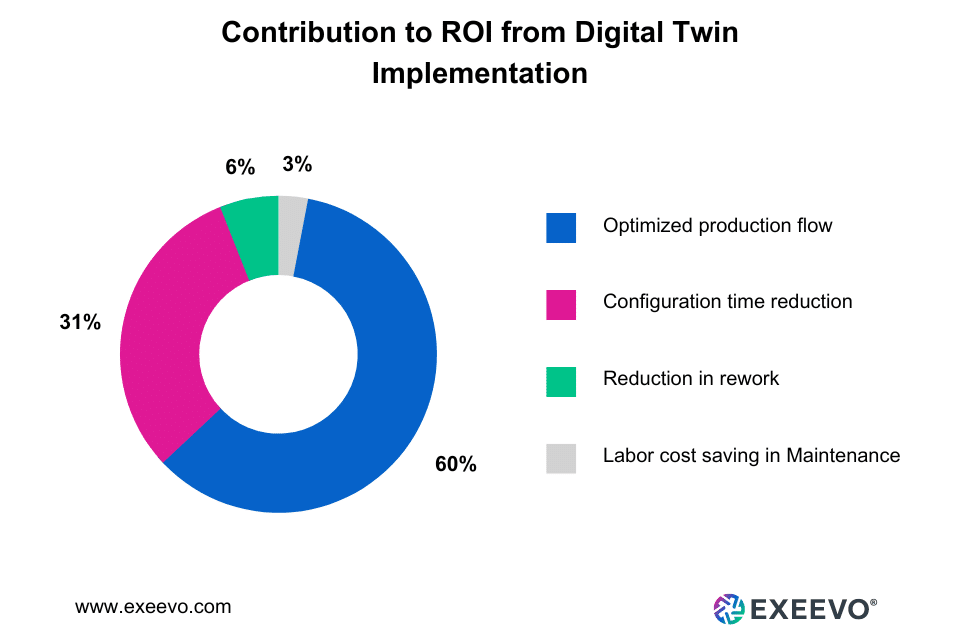
Now, here are a couple of examples that showcase the successful implementation of digital twins in Life Sciences:
- Pharmaceutical cannabis producers used virtual reality-based digital twins that allowed them to act immediately in case of expected deviations and to simulate the impact of different environmental conditions on the growth of the plants.
- A global pharmaceutical company used a digital twin to help identify the millions of patients not receiving treatment for a particular disease. The digital twin created a virtual representation of the human body to model the impact of a particular therapy on patients.
Automation
Process automation speeds up and improves the delivery of crucial medicines and devices worldwide. Robotic process automation (RPA) and intelligent automation are two types of automation that can help achieve this goal.
- RPA: This technology automates repetitive and rule-based tasks to help employees focus on more complex and value-added work.
- Intelligent automation: This type of automation combines RPA with AI and ML to make machines handle tasks that require decision-making and judgment.
Some examples of automated processes and their benefits include:
- High-throughput screening: Optimizes drug development processes by automatically testing a large number of biological modulators against selected targets.
- Automated lab processes: Reduces human error and increases reproducibility in experiments for more reliable outcomes.
- AI in clinical trials: Automates patient recruitment and data collection to speed up the trials and reduce costs.
Key Takeaway

To drive successful digital transformation in life sciences, it is crucial to establish strong relationships with key stakeholders such as researchers and clinicians. This involves fostering collaboration between IT departments and other teams, encouraging open communication channels among different departments, engaging regulatory authorities like the FDA regarding compliance requirements related to emerging technologies, and involving patient advocacy groups in discussions about potential benefits associated with personalized medicine approaches enabled by AI-driven analytics.
How to Start Building a Life Sciences Digital Strategy?
As leaders in the Life Sciences industry recognize the value and necessity of digital transformation, the question arises: How do they start building a successful digital strategy?
The answer lies in implementing a carefully crafted plan specifically tailored for each organization. The strategies below guide the digital transformation process to ensure the transition is smooth, beneficial, and aligned with the organization’s vision and goals.
However, once the transformation process is set in motion, it becomes a continuous journey, not a one-time effort.
Assess the Current Digitalization
Your organization needs to evaluate its current digitalization initiatives and capabilities to shape a robust digital transformation strategy. They must examine how digital technologies are deployed and utilized.
You need to know how advanced the company is in adopting and integrating digital technologies. This understanding highlights areas of strength and gaps to address.
Here’s a checklist for a comprehensive assessment:
- Technology Inventory: Identify all technologies currently in use.
- Digital Skills: Assess the digital proficiency of your team.
- Data Management: Evaluate your data collection, storage, and analysis capabilities.
- Cybersecurity: Check the robustness of your security systems.
- Digital Culture: Understand the organization’s readiness to embrace digital change.
- Customer Experience: Measure the digital experience you offer your customers.
This checklist gives you a holistic view of their digital status. It lays the groundwork for an effective digital transformation journey.
Key Takeaway:

Life sciences industry faces unique regulatory compliance challenges due to complexities surrounding data privacy or patient consent requirements. CIOs must navigate these concerns effectively when leading their organizations through successful digital transformations by implementing robust security measures and creating guidelines that ensure responsible use of AI systems within their organization while promoting transparency in the development and deployment of AI algorithms.
Set Value Metrics
Setting value metrics is crucial to understand the success of digital transformation as it allows Life Sciences companies to measure progress and impact effectively.
Key performance indicators (KPIs) and metrics relevant to the Life Sciences industry might include:
- Operational efficiency
- Employee engagement
- Customer engagement
- Revenue growth
- Cost savings
- Market share
- Time to market
- Mobile and online engagement
- Data-driven decision-making
- Digital adoption
For defining and tracking value metrics, Life Sciences companies should be clear, quantifiable, and directly linked to their objectives. Regularly monitoring and updating these metrics ensures that the digital transformation efforts stay on track and contribute to the intended goals.
Identify the Proper Strategy
Your organization should identify a digital transformation strategy that fits its needs based on its goals, resources, and digital maturity. This strategy should map out the transformation journey and provide details on the required tech adoption and changes.
Some of the considerations could include:
- Cloud Adoption: Moving data and applications to cloud-based platforms can boost accessibility, collaboration, and data security. For instance, using cloud services for genomic data storage and analysis can expedite research in Life Sciences.
- Data Analytics Implementation: The enhanced computational capabilities of data analytics can transform raw data into actionable insights that help with decision-making. For example, using predictive analytics in drug discovery can improve success rates.
- AI Integration: AI can automate tasks, enhance data analysis, and provide advanced capabilities. In medical diagnostics, AI can help rapidly and accurately identify diseases.
You must weigh these options against their needs, resources, and readiness to ensure a successful digital transformation.
Develop an Operating Model
A robust operating model is crucial to support digital transformation because it serves as a blueprint for organizing and operating resources. Aligning processes, roles, and responsibilities within this model ensures that every part of the organization works towards the shared objective of digital transformation.
Creating an operating model for a patient-centric approach in the Life Sciences industry involves several key steps:
- Secure Leadership Commitment: Secure buy-in from leadership and appoint Chief Patient Officers with global responsibilities.
- Define Clear Goals: Establish clear, realistic objectives for patient centricity.
- Eliminate Silos: Break down organizational silos and centralize operations around patient focus.
- Form Strategic Partnerships: Collaborate with diagnostics, technology companies, service providers, and payers for optimal results.
- Invest in Technology: Deploy centralized hubs, digital apps, data tracking, and seamless communication channels for patient support.
- Improve Collaboration: Develop organization-wide strategies to pull insights from various data sources and improve patient experience.
Key Takeaway:

Life sciences organizations need to embrace agility and adaptability during digital transformation efforts in order to navigate industry challenges and maintain long-term success. Agility enables quick responses and adaptation to changes, while adaptability allows for adjustments in overall strategy when faced with unexpected obstacles or opportunities. Data-driven decision-making, continuous learning, and cross-functional collaboration are key factors for successful digital transformation in the life sciences sector.
Understanding the Digital Landscape in Life Sciences
Several key technologies are shaping the digital landscape in the Life Sciences industry. Some of them include:
- Cloud Computing: Cloud technology offers scalable resources and data storage that facilitate collaboration and real-time data access. For example, Novartis uses the cloud to optimize operations and accelerate innovation.
- Artificial Intelligence (AI): AI and machine learning algorithms can quickly analyze vast amounts of data and deliver valuable insights. For example, Deep Genomics leverages AI for gene therapy development to quickly develop drugs to treat genetic diseases.
- Big Data Analytics: The ability to analyze and interpret large volumes of data can lead to breakthroughs in research and development. Firms like Flatiron Health use big data analytics to accelerate cancer research and improve treatment.
These technologies have the potential to streamline processes, amplify research capabilities, and significantly enhance patient care.
Developing a Digital Transformation Strategic Framework
Building a strategic framework for digital transformation is a crucial step for C-suite executives in the Life Sciences sector. This framework serves as a roadmap that aligns digital initiatives with business objectives and customer needs.
- Define Objectives: Start by clearly defining what you want to achieve. These objectives should align with your business goals, whether you want to boost operational efficiency, improve customer experience, or foster innovation.
- Assess Digital Maturity: Evaluate your organization’s current digital capabilities. Identify gaps, strengths, and areas for improvement.
- Prioritize Initiatives: Prioritize digital initiatives based on their potential impact, feasibility, and alignment with business objectives.
- Develop an Action Plan: Create a detailed plan of action that specifies the tasks, timelines, responsibilities, and resources you need.
- Implement, Measure, and Adjust: Implement the plan, measure progress using predefined metrics, and adjust as necessary.
Your organization can craft this strategic digital transformation framework to streamline the transition process, effectively manage resources, and ensure that digital investments yield desired outcomes.
Fostering Collaboration Between IT Departments and Stakeholders
Collaboration between IT departments and stakeholders, such as researchers or clinicians, is crucial for success in digital transformation. It ensures technology initiatives align with business goals and promotes seamless integration of new digital strategies across all departments.
Cross-functional collaboration can spark new ideas, solve problems collectively, and help ensure everyone is on the same page during the rollout of digital changes.
Organizations can consider these strategies to foster effective collaboration:
- Shared Vision: Establish a shared vision of the digital transformation journey. This shared understanding aligns goals and efforts across the organization.
- Cross-Functional Teams: Encourage the formation of cross-functional teams comprising members from the IT department and other relevant departments. Such teams can provide diverse perspectives, promoting innovation.
- Communication Channels: Implement effective communication channels. Regular meetings, updates, and open dialogues ensure everyone is on the same page and can promptly address challenges.
With strong collaboration, organizations can harness the power of collective intelligence to navigate their digital transformation journey successfully.
Key Takeaway:

To succeed in digital transformation, life sciences companies must align their objectives with customer preferences and business goals. This involves retraining employees on necessary digital skills and co-creating solutions that address problems while delivering value through cross-functional collaboration, a patient-centric approach, and implementing real-time feedback loops. By doing so, organizations can create a competitive advantage in the industry landscape while improving patient outcomes and driving business success.
Navigating Regulatory Compliance and Ethical Considerations
The Life Sciences industry has to deal with special rules around data privacy and patient permission. There are legal issues linked to new technologies like AI or IoT devices, and CIOs need to handle these well.
Digital transformation also brings unique challenges, such as potential data breaches, misuse of sensitive information, and ensuring compliance with evolving regulations.
To maintain compliance while embracing digital transformation, consider these insights:
- Data Governance: Establish robust data governance policies. This includes procedures for data collection, storage, usage, and disposal as per industry regulations.
- Regular Audits: Conduct regular audits of your digital processes to identify potential risks or non-compliance issues. Early detection allows for prompt remedial action.
- Staff Training: Educate your staff about regulatory and ethical standards. A well-informed team is a critical line of defense against breaches and non-compliance.
Organizations can prioritize regulatory compliance and ethical considerations to drive digital transformation while preserving their reputation and integrity.
Embracing Agility and Adaptability During Digitalization Efforts
Embracing agility and adaptability is key to successful digital transformation. The rapid pace of technological change requires organizations to be flexible and responsive.
- Embrace Change: Stay open to new technologies and ways of working. Foster a culture that sees change as an opportunity, not a threat.
- Overcome Resistance: Address resistance to change through clear communication and training. Involve employees in the transformation process to gain their buy-in.
- Stay Competitive: Stay ahead of competitors by continuously learning and adapting. Keep up with industry trends and technological advances.
Examples of agility and adaptability in digital transformation abound. For instance,
- Pharmaceutical giant Roche adopted a digital-first approach. They embraced data analytics, cloud technology, and AI to streamline and enhance various processes.
- Medtronic, a medical technology company, pivoted to digital solutions such as AI, IoT, and data analytics to improve patient outcomes and operational efficiency. Their agile and adaptable approach was key to their successful digital transformation.
Aligning Digital Transformation with Customer Experience
Aligning digital transformation with customer experience is essential for the Life Sciences industry. Digital solutions can significantly boost customer satisfaction and loyalty by enhancing customer interactions.
CRM software like Exeevo’s Omnipresence plays a pivotal role in this alignment. As an omnichannel solution, now 365 Copilot enabled, it provides an orchestrated, unified view of customer interactions, enabling personalized experiences and streamlined communication.
There are three important factors that can help you effectively leverage digital solutions to enhance the customer experience:
- Understand Customer Needs: Use data analytics to discover insight into customer preferences and behaviors and tailor your offerings accordingly.
- Personalize Interactions: Utilize CRM software to personalize customer interactions. Personalization can significantly enhance customer satisfaction and loyalty.
- Improve Accessibility: Make your services more accessible through digital platforms. The easier it is for customers to access your services, the better their experience will be.
As Life Sciences companies align digital transformation with customer experience, they can improve customer satisfaction and foster long-term customer relationships.
Final Thoughts
Digital transformation in the Life Sciences industry is not a trend; it’s a strategic step. It enables organizations to optimize processes, enhance research, and significantly improve patient care. Embracing technologies like AI, cloud computing and data analytics while leveraging solutions like Exeevo’s Omnipresence CRM can unlock tremendous potential for growth and innovation.
Organizations must navigate challenges related to regulatory compliance and cybersecurity, foster collaboration between IT departments and stakeholders, and ensure alignment of digital transformation initiatives with customer experience. Agility, adaptability, and a well-defined strategic framework are crucial for successfully navigating the transformation journey.
Whether you’re just beginning your digital transformation or are looking to optimize ongoing efforts, Exeevo is a trusted partner in facilitating this critical shift in the Life Sciences industry.
Get in touch with us to learn how Exeevo’s solutions recognized by Gartner, IDC and Everest as the only alternative to Salesforce-based systems can drive your digital transformation success. You can also explore more insights on our blog page.
If you’re looking to drive your organization’s digital transformation forward within the life sciences industry, then Exeevo can help!
